Anterior hippocampus: the anatomy of perception, imagination and episodic memory
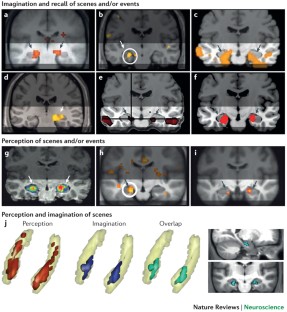
The brain creates a model of the world around us. We can use this representation to perceive and comprehend what we see at any given moment, but also to vividly re-experience scenes from our past and imagine future (or even fanciful) scenarios. Recent work has shown that these cognitive functions — perception, imagination and recall of scenes and events — all engage the anterior hippocampus. In this Opinion article, we capitalize on new findings from functional neuroimaging to propose a model that links high-level cognitive functions to specific structures within the anterior hippocampus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
186,36 € per year
only 15,53 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
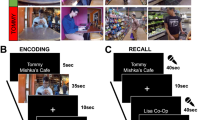
Flexible reuse of cortico-hippocampal representations during encoding and recall of naturalistic events
Article Open access 08 March 2023
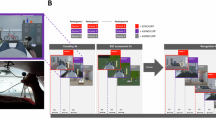
Embodiment in episodic memory through premotor-hippocampal coupling
Article Open access 10 September 2024

The influence of the precuneus on the medial temporal cortex determines the subjective quality of memory during the retrieval of naturalistic episodes
Article Open access 04 April 2024
References
- Fanselow, M. S. & Dong, H.-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron65, 7–19 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Poppenk, J., Evensmoen, H. R., Moscovitch, M. & Nadel, L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci.17, 230–240 (2013). ArticlePubMedGoogle Scholar
- Strange, B. A., Witter, M. P., Lein, E. S. & Moser, E. I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci.15, 655–669 (2014). ArticleCASPubMedGoogle Scholar
- Moser, M.-B. & Moser, E. I. Functional differentiation in the hippocampus. Hippocampus8, 608–619 (1998). ArticleCASPubMedGoogle Scholar
- Chase, H. W. et al. Evidence for an anterior–posterior differentiation in the human hippocampal formation revealed by meta-analytic parcellation of fMRI coordinate maps: focus on the subiculum. Neuroimage113, 44–60 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Yushkevich, P. A. et al. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage111, 526–541 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Scoville, W. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry20, 11–21 (1957). ArticleCASPubMedPubMed CentralGoogle Scholar
- Andelman, F., Hoofien, D., Goldberg, I., Aizenstein, O. & Neufeld, M. Y. Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase16, 426–435 (2010). ArticlePubMedGoogle Scholar
- Maguire, E. A., Nannery, R. & Spiers, H. J. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain129, 2894–2907 (2006). ArticlePubMedGoogle Scholar
- Ding, S.-L. & Van Hoesen, G. W. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemo-architecture. J. Comp. Neurol.523, 2233–2253 (2015). ArticlePubMedGoogle Scholar
- Suzuki, W. A. & Baxter, M. G. Memory, perception, and the medial temporal lobe: a synthesis of opinions. Neuron61, 678–679 (2009). ArticleCASPubMedGoogle Scholar
- Ding, S.-L. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. J. Comp. Neurol.521, 4145–4162 (2013). ArticlePubMedGoogle Scholar
- Rosene, D. L. & Van Hoesen, G. W. The hippocampal formation of the primate brain. Cereb. Cortex6, 345–456 (1987). ArticleGoogle Scholar
- McLardy, T. Some cell and fibre peculiarities of uncal hippocampus. Prog. Brain Res.3, 71–88 (1963). ArticleGoogle Scholar
- Amaral, D. G. & Insausti, R. in The Human Nervous System 1st edn (ed. Paxinos, R.) 711–755 (Academic Press, 1990). BookGoogle Scholar
- Kondo, H., Lavenex, P. & Amaral, D. G. Intrinsic connections of the Macaque monkey hippocampal formation: I. dentate gyrus. J. Comp. Neurol.511, 497–520 (2008). ArticlePubMedPubMed CentralGoogle Scholar
- Kondo, H., Lavenex, P. & Amaral, D. G. Intrinsic connections of the macaque monkey hippocampal formation: II. CA3 connections. J. Comp. Neurol.515, 349–377 (2009). PubMedPubMed CentralGoogle Scholar
- Amaral, D. G., Insausti, R. & Cowan, W. M. The commissural connections of the monkey hippocampal formation. J. Comp. Neurol.224, 307–336 (1984). ArticleCASPubMedGoogle Scholar
- Demeter, S., Rosene, D. L. & van Hoesen, G. W. Interhemispheric pathways of the hippocampal formation, presubiculum, and entorhinal and posterior parahippocampal cortices in the rhesus monkey: the structure and organization of the hippocampal commissures. J. Comp. Neurol.233, 30–47 (1985). ArticleCASPubMedGoogle Scholar
- Gloor, P., Salanova, V., Olivier, A. & Quesney, L. F. The human dorsal hippocampal commissure. Brain116, 1249–1273 (1993). ArticlePubMedGoogle Scholar
- O'Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res.34, 171–175 (1971). ArticleCASPubMedGoogle Scholar
- Fyhn, M., Molden, S., Witter, M. P., Moser, E. I. & Moser, M.-B. Spatial representation in the entorhinal cortex. Science305, 1258–1264 (2004). ArticleCASPubMedGoogle Scholar
- Boccara, C. N. et al. Grid cells in pre- and parasubiculum. Nat. Neurosci.13, 987–994 (2010). ArticleCASPubMedGoogle Scholar
- Wiener, S. & Taube, J. S. Head Direction Cells and the Neural Mechanisms of Spatial Orientation (MIT Press, 2005). BookGoogle Scholar
- Dumont, J. R. & Taube, J. S. The neural correlates of navigation beyond the hippocampus. Prog. Brain Res.219, 83–102 (2015). ArticlePubMedGoogle Scholar
- Lever, C., Burton, S., Jeewajee, A., O'Keefe, J. & Burgess, N. Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci.29, 9771–9777 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Spiers, H. J. & Maguire, E. A. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage31, 1826–1840 (2006). ArticlePubMedGoogle Scholar
- Ekstrom, A. et al. Cellular networks underlying human spatial navigation. Nature425, 184–188 (2003). ArticleCASPubMedGoogle Scholar
- Doeller, C. F., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature463, 657–661 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Auger, S. D., Mullally, S. L. & Maguire, E. A. Retrosplenial cortex codes for permanent landmarks. PLoS ONE7, e43620 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kjelstrup, K. B. et al. Finite scale of spatial representation in the hippocampus. Science321, 140–143 (2008). ArticleCASPubMedGoogle Scholar
- Keinath, A., Wang, M. & Wann, E. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus24, 1533–1548 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Marr, D. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B262, 23–81 (1971). ArticleCASGoogle Scholar
- Wikenheiser, A. M. & Redish, D. A. Decoding the cognitive map: ensemble hippocampal sequences and decision making. Curr. Opin. Neurobiol.32, 8–15 (2015). ArticleCASPubMedGoogle Scholar
- Johnson, A. & Redish, A. D. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci.27, 12176–12189 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Pfeiffer, B. E. & Foster, D. J. Hippocampal place-cell sequences depict future paths to remembered goals. Nature497, 74–79 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Foster, D. J. & Wilson, M. A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature440, 680–683 (2006). ArticleCASPubMedGoogle Scholar
- Clark, I. A. & Maguire, E. A. Remembering preservation in hippocampal amnesia. Annu. Rev. Psychol.67, 51–82 (2016). ArticlePubMedGoogle Scholar
- Hassabis, D., Kumaran, D., Vann, S. D. & Maguire, E. A. Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl Acad. Sci. USA104, 1726–1731 (2007). ArticleCASPubMedGoogle Scholar
- Mullally, S. L., Intraub, H. & Maguire, E. A. Attenuated boundary extension produces a paradoxical memory advantage in amnesic patients. Curr. Biol.22, 261–268 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hassabis, D., Kumaran, D. & Maguire, E. A. Using imagination to understand the neural basis of episodic memory. J. Neurosci.27, 14365–14374 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Addis, D. R., Wong, A. T. & Schacter, D. L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia45, 1363–1377 (2007). ArticlePubMedGoogle Scholar
- Addis, D. R., Pan, L., Vu, M.-A. A., Laiser, N. & Schacter, D. L. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia47, 2222–2238 (2009). ArticlePubMedGoogle Scholar
- Addis, D. R., Cheng, T., Roberts, R. P. & Schacter, D. L. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus21, 1045–1052 (2011). ArticlePubMedGoogle Scholar
- Addis, D. R., Knapp, K., Roberts, R. P. & Schacter, D. L. Routes to the past: neural substrates of direct and generative autobiographical memory retrieval. Neuroimage59, 2908–2922 (2012). ArticlePubMedGoogle Scholar
- McCormick, C., St-Laurent, M., Ty, A., Valiante, T. A. & McAndrews, M. P. Functional and effective hippocampal–neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cereb. Cortex25, 1297–1305 (2015). ArticlePubMedGoogle Scholar
- Zeidman, P., Mullally, S. & Maguire, E. A. Constructing, perceiving, and maintaining scenes: hippocampal activity and connectivity. Cereb. Cortex25, 3836–3855 (2015). ArticlePubMedGoogle Scholar
- Bonnici, H. M. et al. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J. Neurosci.32, 16982–16991 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gaesser, B., Spreng, R. N., McLelland, V. C., Addis, D. R. & Schacter, D. L. Imagining the future: evidence for a hippocampal contribution to constructive processing. Hippocampus23, 1150–1161 (2013). ArticlePubMedGoogle Scholar
- Zeidman, P., Lutti, A. & Maguire, E. A. Investigating the functions of subregions within anterior hippocampus. Cortex73, 240–256 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Hassabis, D. & Maguire, E. A. Deconstructing episodic memory with construction. Trends Cogn. Sci.11, 299–306 (2007). ArticlePubMedGoogle Scholar
- Maguire, E. A. & Mullally, S. L. The hippocampus: a manifesto for change. J. Exp. Psychol. Gen.142, 1180–1189 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Spreng, N. R., Mar, R. A. & Kim, A. S. N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci.21, 489–510 (2009). ArticlePubMedGoogle Scholar
- Svoboda, E., McKinnon, M. C. & Levine, B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia44, 2189–2208 (2006). ArticlePubMedPubMed CentralGoogle Scholar
- Schacter, D. L. et al. The future of memory: remembering, imagining, and the brain. Neuron76, 677–694 (2012). ArticleCASPubMedGoogle Scholar
- Buckner, R. L. & Carroll, D. C. Self-projection and the brain. Trends Cogn. Sci.11, 49–57 (2007). ArticlePubMedGoogle Scholar
- Mullally, S. L. & Maguire, E. A. Counterfactual thinking in patients with amnesia. Hippocampus24, 1261–1266 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Squire, L. R. & Zola-Morgan, S. The medial temporal lobe memory system. Science253, 1380–1386 (1991). ArticleCASPubMedGoogle Scholar
- Lee, A. C., Barense, M. D. & Graham, K. S. The contribution of the human medial temporal lobe to perception: bridging the gap between animal and human studies. Q. J. Exp. Psychol. B58, 300–325 (2005). ArticlePubMedGoogle Scholar
- Murray, E. A., Bussey, T. J. & Saksida, L. M. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci.30, 99–122 (2007). ArticleCASPubMedGoogle Scholar
- Lee, A. C. H. et al. Perceptual deficits in amnesia: challenging the medial temporal lobe “mnemonic” view. Neuropsychologia43, 1–11 (2005). ArticlePubMedGoogle Scholar
- Lee, A. C. H. et al. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus15, 782–797 (2005). ArticlePubMedGoogle Scholar
- Shrager, Y., Gold, J. J., Hopkins, R. O. & Squire, L. R. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J. Neurosci.26, 2235–2240 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hartley, T. et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus17, 34–48 (2007). ArticlePubMedPubMed CentralGoogle Scholar
- Maguire, E. A., Intraub, H. & Mullally, S. L. Scenes, spaces, and memory traces: what does the hippocampus do? Neuroscientisthttp://dx.doi.org/10.1177/1073858415600389 (2015).
- Binder, J. R., Bellgowan, P. S. F., Hammeke, T. A., Possing, E. T. & Frost, J. A. A comparison of two fMRI protocols for eliciting hippocampal activation. Epilepsia46, 1061–1070 (2005). ArticlePubMedGoogle Scholar
- Howard, L. R., Kumaran, D., Ólafsdóttir, H. F. & Spiers, H. J. Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. J. Neurosci.31, 5253–5261 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lee, A. C., Scahill, V. L. & Graham, K. S. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb. Cortex18, 683–696 (2008). ArticlePubMedGoogle Scholar
- Lee, A. C. H., Brodersen, K. H. & Rudebeck, S. R. Disentangling spatial perception and spatial memory in the hippocampus: a univariate and multivariate pattern analysis fMRI study. J. Cogn. Neurosci.25, 534–546 (2013). ArticlePubMedGoogle Scholar
- Aly, M., Ranganath, C. & Yonelinas, A. P. Detecting changes in scenes: the hippocampus is critical for strength-based perception. Neuron78, 1127–1137 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Aly, M. & Yonelinas, A. P. Bridging consciousness and cognition in memory and perception: evidence for both state and strength processes. PLoS ONE7, e30231 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Intraub, H. & Richardson, M. Wide-angle memories of close-up scenes. J. Exp. Psychol. Learn. Mem. Cogn.15, 179–187 (1989). ArticleCASPubMedGoogle Scholar
- Kim, S., Dede, A. J. O., Hopkins, R. O. & Squire, L. R. Memory, scene construction, and the human hippocampus. Proc. Natl Acad. Sci. USA112, 4767–4772 (2015). ArticleCASPubMedGoogle Scholar
- Chadwick, M., Mullally, S. & Maguire, E. A. The hippocampus extrapolates beyond the view in scenes: an fMRI study of boundary extension. Cortex49, 2067–2079 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Race, E., Keane, M. M. & Verfaellie, M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J. Neurosci.31, 10262–10269 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Robinson, J. L. et al. Neurofunctional topography of the human hippocampus. Hum. Brain Mapp.36, 5018–5037 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Blessing, E. M., Beissner, F., Schumann, A., Brünner, F. & Bär, K. J. A data-driven approach to mapping cortical and subcortical intrinsic functional connectivity along the longitudinal hippocampal axis. Hum. Brain Mapp.37, 462–476 (2016). ArticlePubMedGoogle Scholar
- Kondo, H., Saleem, K. S. & Price, J. L. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol.493, 479–509 (2005). ArticlePubMedGoogle Scholar
- Woollett, K. & Maguire, E. A. Acquiring “the knowledge” of London's layout drives structural brain changes. Curr. Biol.21, 2109–2114 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chebat, D.-R. et al. Alterations in right posterior hippocampus in early blind individuals. Neuroreport18, 329–333 (2007). ArticlePubMedGoogle Scholar
- Leporé, N. et al. Pattern of hippocampal shape and volume differences in blind subjects. Neuroimage46, 949–957 (2009). ArticlePubMedPubMed CentralGoogle Scholar
- Fortin, M. et al. Wayfinding in the blind: larger hippocampal volume and supranormal spatial navigation. Brain131, 2995–3005 (2008). ArticlePubMedGoogle Scholar
- Insausti, R. & Muñoz, M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis). Eur. J. Neurosci.14, 435–451 (2001). ArticleCASPubMedGoogle Scholar
- Blatt, G. J. & Rosene, D. L. Organization of direct hippocampal efferent projections to the cerebral cortex of the rhesus monkey: projections from CA1, prosubiculum, and subiculum to the temporal lobe. J. Comp. Neurol.392, 92–114 (1998). ArticleCASPubMedGoogle Scholar
- Maass, A. et al. Laminar activity in the hippocampus and entorhinal cortex related to novelty and episodic encoding. Nat. Commun.5, 5547 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hafting, T., Fyhn, M., Molden, S., Moser, M. B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature436, 801–806 (2005). ArticleCASPubMedGoogle Scholar
- Frings, L. et al. Lateralization of hippocampal activation differs between left and right temporal lobe epilepsy patients and correlates with postsurgical verbal learning decrement. Epilepsy Res.78, 161–170 (2008). ArticlePubMedGoogle Scholar
- Barbas, H. & Blatt, G. J. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus5, 511–533 (1995). ArticleCASPubMedGoogle Scholar
- Fudge, J. L., DeCampo, D. M. & Becoats, K. T. Revisiting the hippocampal–amygdala pathway in primates: association with immature-appearing neurons. Neuroscience212, 104–119 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Aggleton, J. P., Wright, N., Rosene, D. L. & Saunders, R. C. Complementary patterns of direct amygdala and hippocampal projections to the macaque prefrontal cortex. Cereb. Cortex25, 4351–4373 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Maguire, E. A. Memory consolidation in humans: new evidence and opportunities. Exp. Physiol.99, 471–486 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Nieuwenhuis, I. L. C. & Takashima, A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res.218, 325–334 (2011). ArticlePubMedGoogle Scholar
- Aggleton, J. P. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci. Biobehav. Rev.36, 1579–1596 (2012). ArticlePubMedGoogle Scholar
- Duvernoy, H. M. The Human Hippocampus: An Atlas of Applied Anatomy (JF Bergmann, 1988). BookGoogle Scholar
- Boubela, R. N. et al. fMRI measurements of amygdala activation are confounded by stimulus correlated signal fluctuation in nearby veins draining distant brain regions. Sci. Rep.5, 10499 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Winocur, G. & Moscovitch, M. Memory transformation and systems consolidation. J. Int. Neuropsychol. Soc.17, 766–780 (2011). ArticlePubMedGoogle Scholar
- Nadel, L., Winocur, G., Ryan, L. & Moscovitch, M. Systems consolidation and the hippocampus: two views. Debates Neurosci.1, 55–66 (2007). ArticleGoogle Scholar
- Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol.7, 217–227 (1997). ArticleCASPubMedGoogle Scholar
- Byrne, P., Becker, S. & Burgess, N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev.114, 340–375 (2007). ArticlePubMedPubMed CentralGoogle Scholar
- Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature489, 391–399 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Fischl, B. et al. Predicting the location of entorhinal cortex from MRI. Neuroimage47, 8–17 (2009). ArticlePubMedPubMed CentralGoogle Scholar
- Nieuwenhuys, R., Huijzen, C. & Voogd, J. The Human Central Nervous System (Springer, 2008). BookGoogle Scholar
- Nogueira, A. B. et al. Existence of a potential neurogenic system in the adult human brain. J. Transl. Med.12, 75 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hassabis, D. & Maguire, E. A. The construction system of the brain. Phil. Trans. R. Soc. B364, 1263–1271 (2009). ArticlePubMedGoogle Scholar
- Schacter, D. L. & Addis, D. R. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Phil. Trans. R. Soc. B362, 773–786 (2007). ArticleGoogle Scholar
- Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA98, 676–682 (2001). ArticleCASPubMedGoogle Scholar
- Buckner, R. L., Roffman, J. L. & Smoller, J. W. Brain Genomics Superstruct Project (GSP). Harvard Dataverse V10[online], (2014). Google Scholar
- Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol.106, 1125–1165 (2011). ArticlePubMedGoogle Scholar
- Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods8, 665–670 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Spreng, R. N., Sepulcre, J., Turner, G. R., Stevens, W. D. & Schacter, D. L. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J. Cogn. Neurosci.25, 74–86 (2013). ArticlePubMedGoogle Scholar
- Hayes, S. M., Salat, D. H. & Verfaellie, M. Default network connectivity in medial temporal lobe amnesia. J. Neurosci.32, 14622–14629 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Bannerman, D. M. et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci.15, 181–192 (2014). ArticleCASPubMedGoogle Scholar
- Pohlack, S. T., Nees, F., Ruttorf, M., Schad, L. R. & Flor, H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol. Psychol.91, 74–80 (2012). ArticlePubMedGoogle Scholar
- Marschner, A., Kalisch, R., Vervliet, B., Vansteenwegen, D. & Büchel, C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J. Neurosci.28, 9030–9036 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Nees, F. & Pohlack, S. T. Functional MRI studies of the hippocampus. Front. Neurol. Neurosci.34, 85–94 (2014). ArticlePubMedGoogle Scholar
- Li, G., Fang, L., Fernández, G. & Pleasure, S. J. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron78, 658–672 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Perera, T. D. et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS ONE6, e17600 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Maller, J. J., Daskalakis, Z. J. & Fitzgerald, P. B. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus17, 1023–1027 (2007). ArticlePubMedGoogle Scholar
- Leary, O. F. O. & Cryan, J. F. A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol. Sci.35, 675–687 (2014). ArticleCASGoogle Scholar
- Köhler, S., Crane, J. & Milner, B. Differential Contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus12, 718–723 (2002). ArticlePubMedGoogle Scholar
- Poppenk, J., McIntosh, A. R., Craik, F. I. M. & Moscovitch, M. Past experience modulates the neural mechanisms of episodic memory formation. J. Neurosci.30, 4707–4716 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kumaran, D. & Maguire, E. A. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus17, 735–748 (2007). ArticlePubMedGoogle Scholar
- Lepage, M., Habib, R. & Tulving, E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus8, 313–322 (1998). ArticleCASPubMedGoogle Scholar
- Martin, V. C., Schacter, D. L., Corballis, M. C. & Addis, D. R. A role for the hippocampus in encoding simulations of future events. Proc. Natl Acad. Sci. USA108, 13858–13863 (2011). ArticleCASPubMedGoogle Scholar
- Chadwick, M. J., Jolly, A. E. J., Amos, D. P., Hassabis, D. & Spiers, H. J. A goal direction signal in the human entorhinal/subicular region. Curr. Biol.25, 87–92 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Viard, A., Doeller, C. F., Hartley, T., Bird, C. M. & Burgess, N. Anterior hippocampus and goal-directed spatial decision making. J. Neurosci.31, 4613–4621 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
Acknowledgements
The authors are supported by The Wellcome Trust. The authors thank J. Ekanayake for helpful discussions about vasculature.






